Determining the Thickness of Aluminum Foil
How does one determine the thickness of Aluminum Foil? My lab partner Jason and I decided to use the formula D = M/LWH to figure it out, given that V = LWH. We first figured out the density of aluminum by using water displacement to find the volume, and a balance to find the mass of a piece of aluminum.
We then figured the density to be 2.59 g/cm^3.
Next, we measured all sides of the aluminum foil, and since it wasn't a perfect square and the lengths and widths varied, we found the mean of each length and width, then multiplied them into the equation with H.
We found the mass of the aluminum foil using a balance, and it turned out to be 0.3 g. Then we plugged the mass and density into the equation above, until the only unknown was the height, or thickness, of the foil, and then solved for the height.
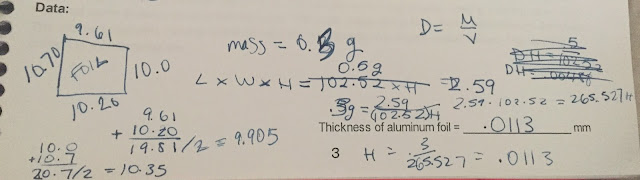 |
| sorry for the messiness |
And that is how I found the thickness of aluminum foil to be approximately .0113 mm!



No comments:
Post a Comment