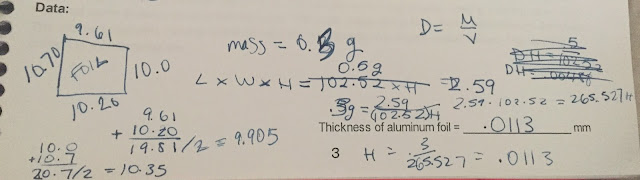What's even cooler than setting stuff on fire in chem class?
Setting stuff on fire in all the colors of the rainbow, duh.
In today's lab, we got to do just that! Ameera, Zoe, and I got to light a bunch of metallic ions on fire to see the different reactions of the excited electrons and what colors were emitted by each. It was so very cool to see blazing flame in such abnormal colors, such as the emerald green, surging lavender, and even the brilliant magenta.
 |
| my personal favorite: copper(II) chloride! |
 |
| love that purple Potassium! |
 |
| Lithium sure is a beaut. |
The answers to the pre-lab questions are below.
We had two unknown metallic ions to test. We figured out that Unkown #1 was Lithium, and Unkown #2 was Potassium. By putting these unknowns in the flames, we were able to discern which metal each was. Since each excited-electron-color-emission is characteristic to each element and no two are the exact same, we matched the unknown colors to the known metal color emissions that we had tested earlier.
 |
| probe |